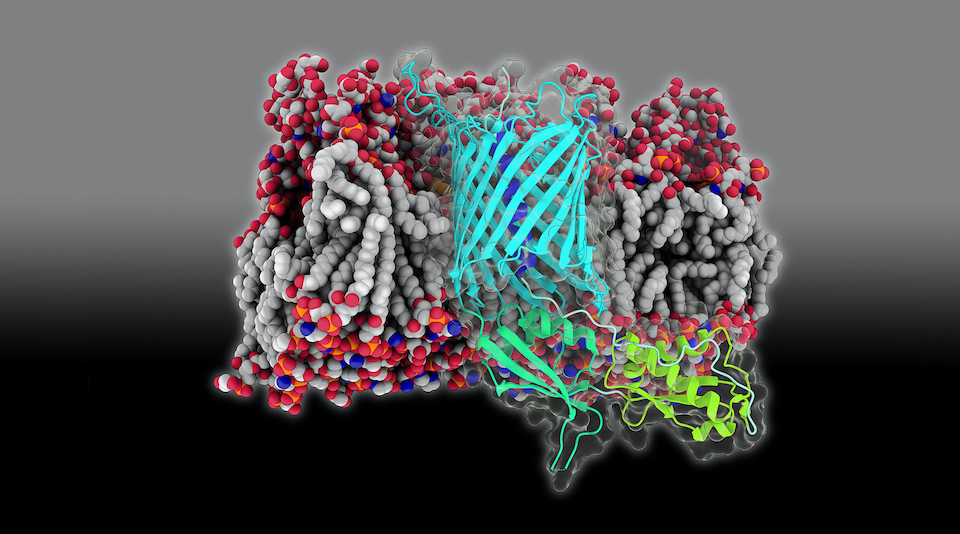
Bacteria are often in battle with each other, fighting over limited resources. One of the weapons they bring into battle is a toxin delivery system known as Contact-Dependent growth Inhibition (CDI). Composed of two proteins, a toxin called CdiA is delivered via a transporter in the bacterial outer membrane called CdiB. In collaboration with structural biologists at NIH, Zijian, Karl, and JC determined how CdiB can be primed for subsequent delivery in a recent paper in eLife. They calculated the energy required to extract the helix that plugs CdiB in its resting state, finding it to be relatively modest. This energy could be provided, for example, by the binding of CdiA prior to its export.